Introduction
♦ ZC1002 PH Sensor is used to measure the hydrogen ion concentration of a solution and display the pH value of the solution.
Usage
♦ Unscrew the pH electrode from protective bottle before using. Connect the electrode to PH Sensor.
♦ The electrode should be cleaned up by using deionized water or distilled water. Its surface should be dried by using tissue. Do not wipe its glass head.
♦ Before measuring, it is required to demarcate the pH electrode and clean the probe.
♦ Screw the pH electrode into the protective bottle carefully after measuring.
Notes
♦ Rinse the electrode before every measuring. Do not wipe its glass ball using tissue. Otherwise it may cause damage to the electrode. It is better to clean the electrode using the testing solution.
♦ Range of measuring temperature: 0~80℃.
♦ Keep the glass ball of the pH electrode wet in order to maintain the ion exchange process. It is required to preserve the glass ball in the KCI solution. Check the KCI solution’s volume periodically. If the electrode dries, immerse it into the KCI solution for 2 hours to make it wet.
♦ Protect the glass ball of the pH electrode. Do not knock, collide or rub it. Otherwise it may cause damage to the electrode.
♦ Keep electrode clean and dry when connecting and unplugging it. If not, it may lead to its insulation failure and make the result not accurate or make the experiment unsuccessful.
♦ We have found that the conductivity sensor has mild interference to this product. Using other devices with it will not cause this problem .
♦ The pH electrode has the limited service life, which mainly depends on its protection and testing solution. Do not store and preserve the electrode in the distilled water or the deionized water. Otherwise it may cause the saturated solution on the electrode to move. Never use the pH electrode in the extreme acid-based or alkaline-based solution or at the extreme temperature.
♦ Do not use the pH electrode in the absolute ethyl alcohol, concentrated sulphuric acid and other dehydration media since they may damage the hydration gel layer on the glass membrane surface. The pH electrode can not either be immersed in the neutral or the alkaline buffer solution. Long-term immersion in such solution may cause the pH glass membrane insensitive to respond.
♦ This product can automatically compensate temperature. User does not need to make temperature compensation.
Typical experiment
Typical experiment
♦ Acid-base neutralization drop
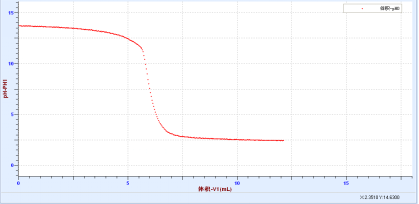
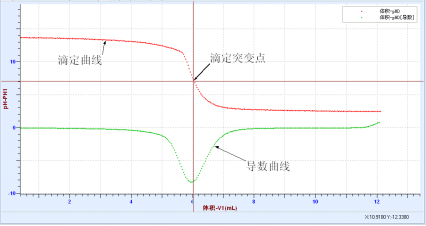
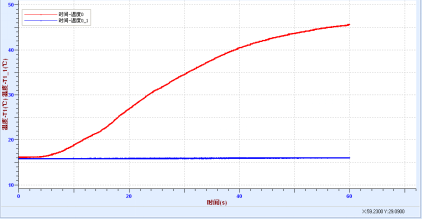
♦ 酸碱中和反应热实验
Others
♦ pH value of different brine solution
♦ Acidity of phenol
♦ Mechanism of organism maintaining pH stability
♦ Explore impact of pH value on pectinase activity



