Introduction
♦ ZC1003 Conductivity Sensor is used to measure the solution’s conductivity and its change.
Usage
♦ The product has two available measuring ranges. Before using, it needs to change the measuring range according to the indication on tag. The red button is pop-up means low measuring range is activated. Press it will become the high measuring range.
♦ Before using, please dip the conductive electrode into the deionized water or distilled water to ensure the electrode is clean. Dry its surface using tissue.
♦ Connect the sensor, datalogger to computer correctly. Open the datalogger and software. Calibrate the sensor using conductivity water. When measuring, fix the electrode well and the front end of the electrode should be completely dipped into the testing solution.
♦ Clean the electrode completely to prevent from pollution after experiment.
Notes
♦ Clean the electrode completely to prevent from pollution after experiment.
♦ Rinse the electrode after using. Dry it using filter paper and preserve it in dry place. The conductivity electrode must be kept clean to avoid sediment and other scales.
♦ Keep the electrode away from the sticky organic liquid such as heavy oil, glycerol, or ethylene glycol. Do not let the electrode contact the non-polar solvent such as acetone, pentane or hexane.
♦ The water soluble pollutant can be removed by the distilled water via immersion. The oily pollutant can be removed by warm water containing a small amount of detergent via immersion for 15 minutes. The ethanol or the acetone can be used to clean the electrode, but the cleaning time should not exceed 5 minutes. Lime or hydroxide cladding can be removed by immersing it into diluted acid solution for 15 minutes. After cleaning, rinse with the distilled water and then leave it dry for storage.
Typical experiment
Typical experiment
♦ Ionization of glacial acetic acid
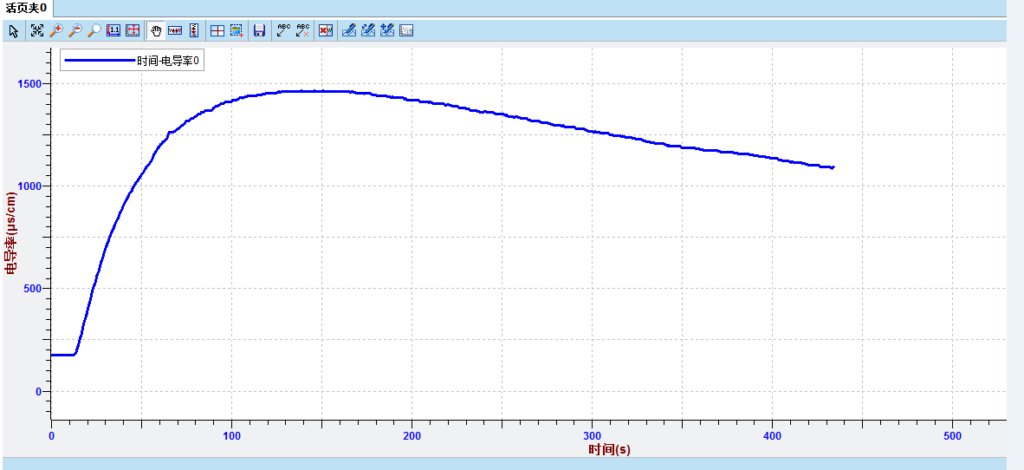
Others
♦ Compare the conductivity of different water
♦ Reaction of phenol and saturated bromine water
♦ Explore the purity of drinking water
♦ Relationship between cell size and translocation of solute


